The two-gene module HEPN/MNT is predicted to be the most abundant toxin/antitoxin (TA) system in prokaryotes. However, its physiological function and neutralization mechanism remains obscure.
Recently, researchers led by Prof. WANG Xiaoxue from the South China Sea Institute of Oceanology (SCSIO) of the Chinese Academy of Sciences found that the MntA antitoxin (MNT-domain protein) acts as an adenylyltransferase and chemically modifies the HepT toxin (HEPN-domain protein) to block its toxicity as an RNase. This work was published in Nucleic Acids Research on Oct. 13.
Researchers from WANG's group have previously shown that MntA antitoxin and HepT toxin form a stable heterooctamer, an organization that is very rare in other TA systems. However, in this study, they showed that polyadenylylation on HepT is the key to inactivate the toxicity.
Biochemical and structural studies revealed that MntA mediates the transfer of three AMPs to a tyrosine residue next to the RNase domain of HepT in Shewanella oneidensis.
"In vitro enzymatic assays showed that the three AMPs are transferred to HepT by MntA consecutively with ATP serving as the substrate, and this polyadenylylation is crucial for reducing HepT toxicity," said Prof. WANG.
Additionally, the GS-X10-DXD motif, which is conserved among MntA proteins, is the key active motif for polyadenylylating and neutralizing HepT. Thus, HepT/MntA represents a new type of TA system, and the polyadenylylation-dependent TA neutralization mechanism is prevalent in bacteria and archaea.
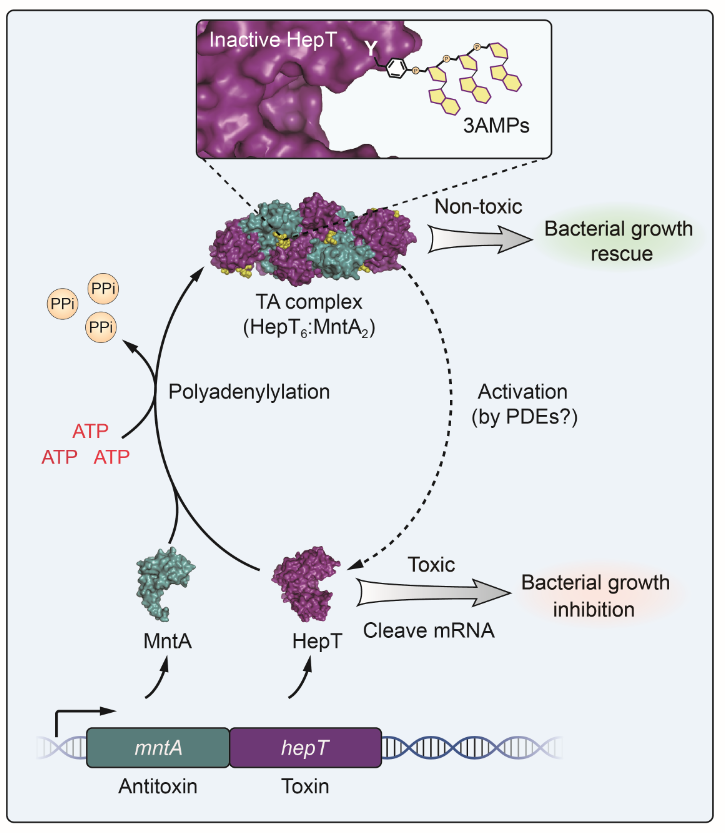
A proposed model of the neutralization mechanism of the HepT/MntA TA system.(Image by YAO Jianyun)
