Recently, a research group in the Key Laboratory of Tropical Marine Bioresources and Ecology (LMB), South China Sea Institute of Oceanology, Chinese Academy of Sciences, reported the structure diversification of bioactive atypical angucyclines by inactivation of redox enzyme-encoding genes in fluostatin biosynthesis. These studies were recently published in The Journal of Organic Chemistry (DOI: 10.1021/acs.joc.0c02517) and Journal of Nature Products (DOI: 10.1021/acs.jnatprod.1c00461). The paper in The Journal of Organic Chemistry was selected as a supplementary cover article in the Special Issue “Natural Products: An Era of Discovery in Organic Chemistry” of August 20, 2021.
Biosynthetic redox enzymes often play significant roles in bringing structure richness of natural products. In the biosynthetic gene clusters (BGCs) of atypical angucyclines, an important natural products with antibacterial, antitumor and enzyme inhibition activities, a number of redox enzymes were found but their biosynthetic functions were unclear yet. In recent studies, the research group carryout out in vivo gene disruptions of two redox enzyme-encoding genes flsO1 and flsP in the BGC of fluostatins, and reported the structure diversification of fluostatin-related atypical angucyclines by functional manipulation of redox enzymes and their encoding genes.
Bioinformatics analysis reveals that the flsO1 from the fluostatin BGC encodes a flavin oxygenase, which is highly homologous to AlpK, an oxygenase proposed to catalyze hydroxylation of benzofluorene in kinamycin biosynthesis. Researchers inactivated flsO1 by both insertional mutation and in-frame deletion, and found that the resulting mutant lost the ability to produce fluostatins (FSTs), but accumulated five known compounds and four new compounds gephyromycin D (11), 6‐hydroxyfridamycin E (12), fluostarenes A and B (14 and 15). The single X-ray crystal analyses confirmed that both 14 and 15 featured an unprecedented 6/6/5/6/6 pentacyclic skeleton with fusion of a benzo[b]fluorene and a six-membered lactone ring. Fluostarene B (15) was found to exhibit moderate cytotoxicity to tumor cell lines. Interestingly, 14, 15 and the known compound PK1 (16) were shown to have α-glucosidase inhibition activity with IC50 of 0.89, 1.58 and 0.13 μM, respectively.
A following complementation experiments demonstrated that production of fluostatins could be restored by introducing either flsO1 or alpK into the in-frame deletion mutant ?flsO1i. Thus, FlsO1 was hinted to function equivalently to AlpK as a putative C-5 benzofluorene hydroxylase. Biosynthetically, compounds 11?12 and 14?16 were proposed to be shunt products from biosynthetic intermediates in the FST biosynthetic pathway (J Org Chem, 2021, DOI: 10.1021/acs.joc.0c02517).
Bioinformatics analysis shows that the flsP-encoding oxidoreductase was likely involved in the modification of the highly oxygenated A-ring in fluostatins. A subsequent insertional deletion of flsP in Micromonospora rosaria SCSIO N160, the fluostatin producer, led to the discovery of a series of atypical angucyclines derivatives, including a novel heterodimer difluostatin I, five new FSTs devivatives (T-X), and six known compounds FSTs (C, E, F, J, K) and
dehydrorabelomycin. Difluostatin I represents the first example of an A-ring-cleaved 3′,4′-seco-fluostatin skeleton and fluostatin W contains an uncommon isoxazolinone ring. FSTs (T, V and X) exhibited inhibition activity against Candida albicans SC5314. However, the major fluostatin related metabolites (FSTs (C, E, F, J, K)) had comparable yields in the ΔflsP mutant to those in the wild-type strain, making it elusive to explain the function of the oxidoreductase FlsP in fluostatin biosynthesis. (J Nat Prod, 2021, DOI: 10.1021/acs.jnatprod.1c00461)
These studies were mainly carried out by Drs YANG Chunfang and HUANG Chunshuai , and were supervised by Professor ZHANG Changsheng and ZHANG Wenjun and associate professor YANG Chunfang. The research group appreciated financial supports from MOST, NSFC, CAS, Key Science and Technology Project of Hainan Province, the Guangdong Provincial Special Fund for Marine Economic Development Project and Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou).
Paper 1 link: https://pubs.acs.org/doi/10.1021/acs.joc.0c02517.
Supplementary cover link of paper 1: https://pubs.acs.org/toc/joceah/86/16
Paper 2 link: https://pubs.acs.org/doi/10.1021/acs.jnatprod.1c00461
Contact :Prof. ZHANG Changsheng , czhang@scsio.ac.cn

Fig 1. The supplementary cover of paper in The Journal of Organic Chemistry
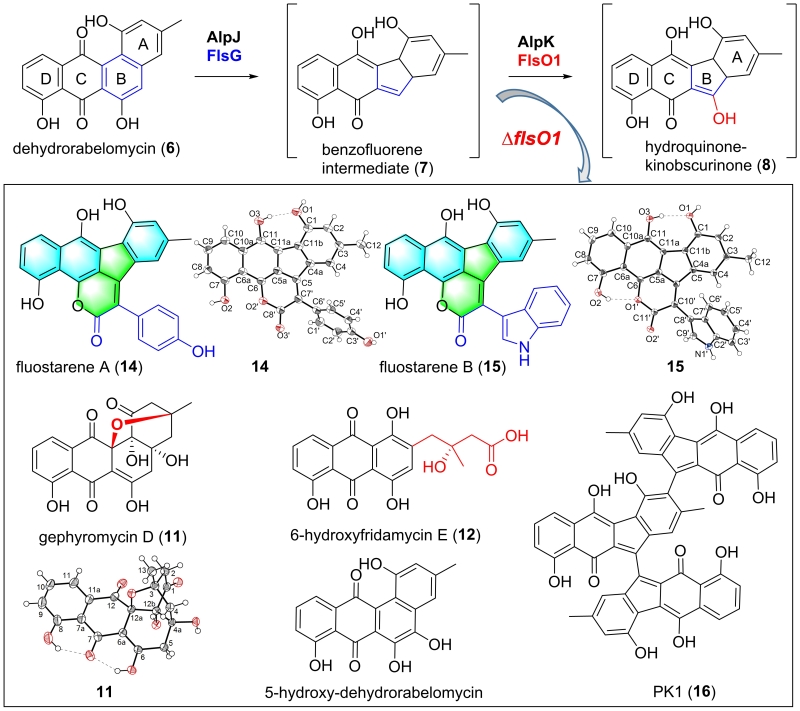
Fig 2. Inactivation of a flavin oxygenase-encoding gene flsO1 leading to novel compounds and proving the function of FlsO1 equivalently to AlpK as a putative C-5 benzofluorene hydroxylase (paper 1)(Image by SCSIO)
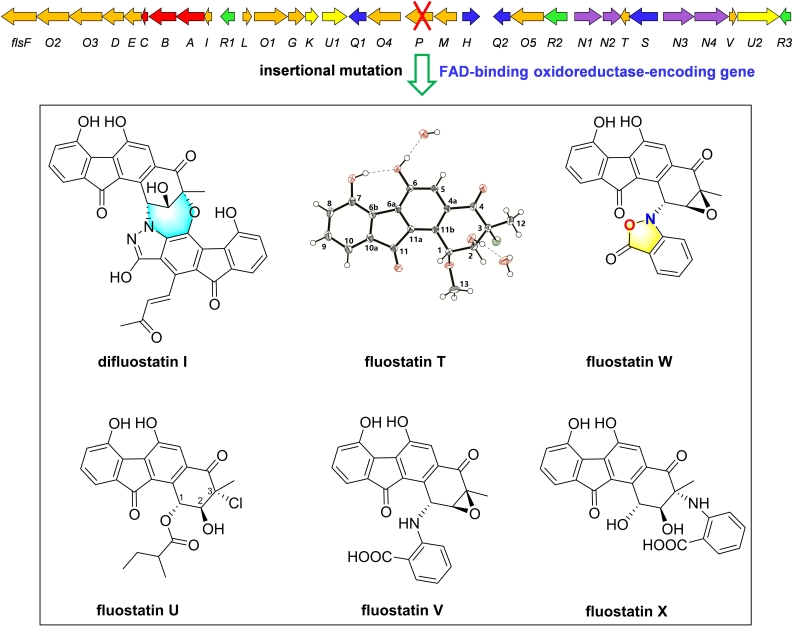
Fig 3. Inactivation of a FAD binding oxidoreductase encoding gene flsP leading to novel FSTs derivatives (paper 2)(Image by SCSIO)
