Chromone derivatives are naturally occurring compounds widely found in plants and fungi, exhibiting potential therapeutic indication. However, few natural dihydrothiophene-condensed chromones were reported. Researchers of South China Sea Institute of Oceanology and Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences, reported the new progress in the structure–activity relationship studies of the antitumor lead compound oxalicumone derivatives (published in the issue of June 1, Bioorganic & Medicinal Chemistry Letters, 2014, 24(11): 2433-2436).
Previous investigations found that the new dihydrothiophene-condensed chromone oxalicumone A showed significant cytotoxicity against several carcinoma cell lines (Planta Medica 2013, 79: 1474-1479). A larger scale fermentation of marine-derived fungus Penicillium oxalicum SCSGAF 0023 led to the isolation of nine chromones including four dihydrothiophene-condensed chromones oxalicumones A-B (1-2) and new oxalicumones D-E (3-4) (Figure 1). Eleven derivatives were obtained by the acylation of 1 and 2, respectively. All compounds were tested for their cytotoxicities against eight carcinoma cell lines. Three of these compounds showed significant cytotoxicity against the eight tested cell lines with IC50 <10 μM. Their structure–bioactivity relationship was further discussed: 2,3-dihydrothiophene unit was most important for the cytotoxicity of these chromones, and the O-methyl groups at C-16 and C-17 were also important, while 13-OH was not necessary for the cytotoxicity of 1; moreover, the absolute configuration of C-6 also had effect on the cytotoxicity of 1.
This work was mainly accomplished by Dr. BAO Jie, and the cytotoxic activity of all compounds was tested by Dr. TU Zhengchao. This work is supported by the Ministry of Science and Technology of China, the State Oceanology Administration, and the National Natural Science Foundation of China.
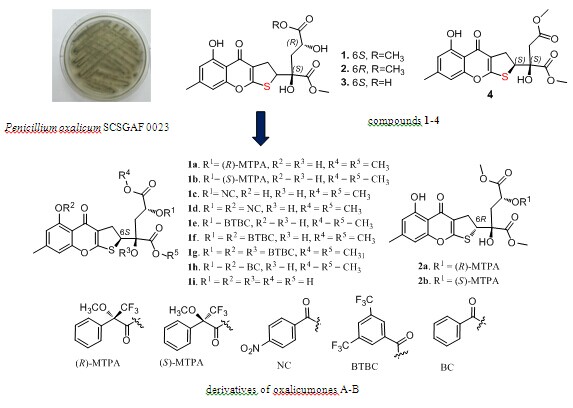
Figure 1. Structures of compounds 1-4 and derivatives of oxalicumones A-B (Image by SCSIO)