Scientists discover AmiG as a reversible retaining glycosyltransferases in natural product biosynthesis
Sugar moieties in many medicinal natural products are often critical for the biological activity, specificity and pharmacological properties. Attachments of sugars to natural product scaffold are catalyzed by glycosyltransferases (GTs) with either inversion or retention of configuration at the anomeric carbon of the donor sugar substrate. Now, scientists from South China Sea Institute of Oceanology,
The disaccharide nucleoside antibiotic amicetin is an antibacterial and antiviral agent, and features a unique a-(1?4)-glycoside bond between amosamine and amicetose, characteristic for a retaining glycosylation. The recent identification and characterization of the biosynthetic gene cluster of amicetin implicated two alternative strategies for amide bond formation, and revealed five gene products are involved in amsoamine biosynthesis (Applied and Environmental Microbiology, 2012, 78, 2393-2401). Further studies revealed that AmiH was an amosamine N-dimethyltransferase, and AmiG as a retaining GT tailoring amicetin biosynthesis. Utilizing genetic, biochemical and kinetic studies, AmiG was demonstrated for the first time as a retaining GT in secondary metabolite biosynthesis to catalyze reverse reaction and to be amenable of sugar and aglycon exchange reactions. Furthermore, AmiG displays certain substrate flexibility capable of accepting five additional non-natural sugar donor substrates, thus being a potential catalyst for the diversification of nucleoside antibiotics. This study also paves the way to investigate the catalytic mechanism of retaining GTs using AmiG as a model.
The research was supported by grants from National Natural Science Foundation of China, Ministry of Science and Technology, and
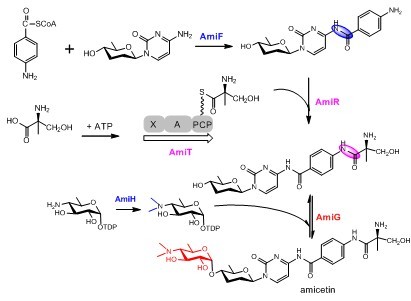
The proposed key biosynthetic steps in amicetin biosynthesis.