Scientists discover key enzymes involved in caerulomycin A biosynthesis
The marine actinomycete-derived Caerulomycin A (CRM A) was currently under the development as an immunosuppressive agent. Structurally, CRM A bears a unique 2,2'-bipyridine core and an unusual oxime functionality, the biosynthesis of which is rarely found in microbial secondary metabolites. Now, scientists from South China Sea Institute of Oceanology revealed that a flavin-dependent, two-component monooxygenase CrmH catalyzed the oxime formation in CRM A biosynthesis (published in the issue of December 18, Journal of the American Chemical Society, 2013, 135, 18750-18753).
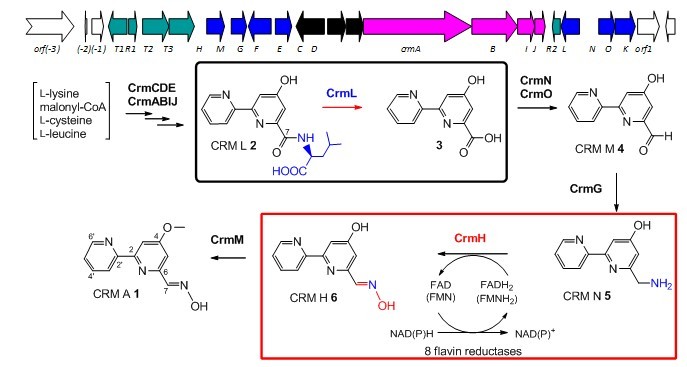
Fig. 1 The proposed CRM A biosynthetic pathway. CrmL-catalyzed amidohydrolysis and CrmH-catalyzed oximation are highlighted in boxes.
Caerulomycin A was isolated from a marine-derived Actinoalloteichus cyanogriseus WH1-2216-6. The recent identification and characterization of the biosynthetic gene cluster of CRM A revealed that the 2,2'-bipyridine core was formed from a hybrid polyketide synthase (PKS)/nonribosomal peptide synthase (NRPS) machinery, involving an unusual protection/deprotection strategy with adding/removing an L-leucine residue by an amidohydrolase CrmL (Organic Letters. 2012, 14, 2666-2669; highlighted as a “Hof off the press” paper in Natural Product Reports, 2012, 29, 829-833). Further genetic and biochemical experiments demonstrate that the oxime formation in CRM A biosynthesis is catalyzed by CrmH, a flavin-dependent two-component monooxygenase that is compatible with multiple flavin-reductases, from a primary amine via N-hydroxylamine intermediate, with spontaneous generation of dimeric byproducts during CrmH-catalysis. In addition, four amino acid residues were identified to be essential for CrmH catalysis by structure homolog-guided site-directed mutagenesis studies. This study provides the first biochemical evidence of a two-component monooxygenase that catalyzes oxime formation, and six flavin-reductases ware discovered as as potentially new tools for characterizing other enzymes requiring flavin reductases (Journal of the American Chemical Society, 2013, 135, 18750-18753).
The research was supported by grants from National Natural Science Foundation of China, Ministry of Science and Technology, and Chinese Academy of Sciences.