Deciphering the Biosynthetic Mechanism of β-Carboline Alkaloid from Deep Sea-Derived Microorganism
The β-carboline (βCs) alkaloids possessing a tricyclic pyrido[3, 4-b]indole ring system are widely distributed in nature; the heterocyclic skeleton endows the βCs with antiallergic, antiviral, anti-inflammatory, antibacterial, and antitumor activities, and enables interactions with various receptors leading to neurotoxin and neuroprotectant activities. However, the enzymes driving biosynthesis of βCs have not yet been deciphered.
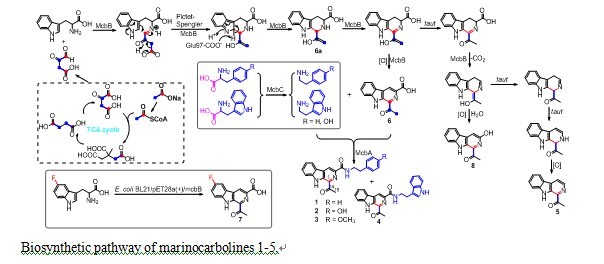
Accordingly, following the isolation of marinocarbolines A–D (MCBs, 1–4) and 1-acetyl-β-carboline (5) from the deep South China Sea-derived Marinactinospora thermotolerans SCSIO 00652, Qi Chen and other team members of Pr. Jianhua Ju’s group identified and elucidated three genes, mcbABC, that drive the biosynthesis of MCBs using genome scanning, bioinformatics analysis, gene inactivation, and heterologous expression in Streptomyces lividans TK64 and Escherichia coli BL21 strategies. The gene, mcbB, is sufficient in E. coli for the production of1-acetyl-3-carboxy-β- carboline (6) as the major product and two other minor products 1-acetyl-β-carboline (5) and 1-acetyl-3-hydroxy-β-carboline (8), thus highlighting it as a novel enzyme for β-carboline scaffold construction. Feeding experiments with 5-F-Trp and 13C-labeled glucose and acetate reveal that 6 is derived from Trp and two equivalents of acetate as precursors. Generation of 6 by McbB involves a Pictet–Spengler cyclization, a decarboxylation and C-ring oxidation process. Site-directed mutagenesis experiments with McbB reveal that E97 is absolutely required for biochemical activity.
The results have published on Angew. Chem. Int. Ed. (2013, 52, 9980-9984). Sooner after its publication, the article was recommended by Faculty of 1000 commenting “This finding is quite valuable because McbB has little amino acid sequence similarity with known PS-catalyzing enzymes, thus indicating that this enzyme is likely to possess a novel protein structure. In addition, because a lot of McbB homologues are distributed among bacteria and fungi, the discovery of McbB will contribute to the elucidation of the biosyntheses of various microbial β-carboline compounds.”
This work was financially supported by grants from MOST, NSFC and CAS.