Regioselectivity improvement of the α/β epoxide hydrolase Alp1U
Epoxide hydrolases (EHs) are a class of important biocatalysts that have been characterized and engineered to convert epoxides to chiral vicinal diol precursors of drugs and bioactive compounds. However, their utility is limited by often producing stereoisomeric products. Up to date, the regioselectivity control of the epoxide ring opening by EHs remains challenging.
Recently, researchers from ZHANG Changsheng’s group in the South China Sea Institute of Oceanology of the Chinese Academy of Sciences found that mutation of an atypical oxirane oxyanion hole could significantly improve regioselectivity of the α/β-fold epoxide hydrolase Alp1U to provide almost optically pure products. This work was published as a latest research in Journal of Biological Chemistry titled “Mutation of an atypical oxirane oxyanion hole improves regioselectivity of the α/β-fold epoxide hydrolase Alp1U” (DOI: 10.1074/jbc.RA120.015563).
Researchers from Zhang’s group have previously shown that the α/β-fold EH Alp1U found in Streptomyces ambofaciens also uses fluostatin (FST) C (1) from marine-derived Micromonospora rosaria as a substrate. However, Alp1U exhibits poor regioselectivity in the epoxide hydrolysis of 1, and produces a pair of stereoisomers, 1a and 1b (Nature Communications, 2018, 9, 2088). In this work (figure 1), they established the absolute configuration of the two stereoisomeric products 1a and 1b and determined the crystal structure of Alp1U. Interestingly, Alp1U structurally shows conserved overall fold as classic α/β-EHs, but features a unique triad (W186/W187/Y247) to assemble an atypical oxirane oxygen hole that is usually defined by two residues of Tyr/Tyr for the canonical α/β-EHs. Astonishingly, mutation of residues in the atypical oxirane oxygen hole of Alp1U improved the regioselectivity for epoxide hydrolysis on 1. The single site Y247F mutation led to highly regioselective (98%) attack at C-3 of 1, while the double mutation W187F/Y247F resulted in regioselective (94%) nucleophilic attack at C-2 (figure 1). To investigate the reaction details of Alp1U, researchers further solved the single crystal X-ray structures of the two regioselective Alp1U variants in complex with the substrate 1. Slight conformation changes of the substrate and residues of the oxirane oxygen hole are observed. These structural and mutagenesis studies of Alp1U and its mutants provided further insight into the factors governing the regioselectivity of α/β-EHs, which suggests that other parameters such as hydrophobic effects should be considered, in addition to the distance of the interacting atoms.
This study was supported by the NSFC, the CAS, the Special Project for Introduced Talents Team of Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou) and Guangdong Province.
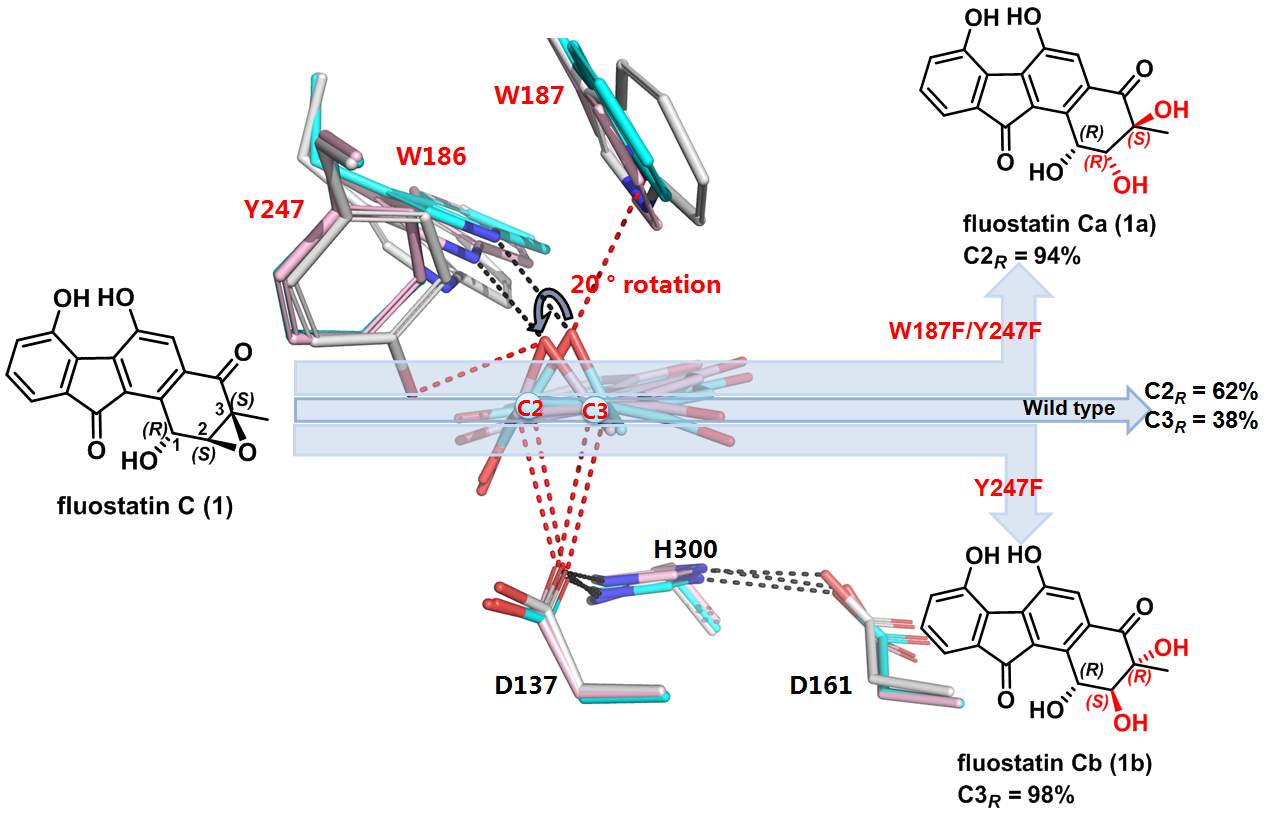
Figure 1. Mutation of an atypical oxirane oxyanion hole improves regioselectivity of the α/β-fold epoxide hydrolase Alp1U.
Paper link: https://www.jbc.org/content/early/2020/10/01/jbc.RA120.015563.abstract